STUDIES ON THE ECOLOGY OF ALDROVANDA VESICULOSA L.I. ECOLOGICAL DIFFERENTIATION OF A. VESICULOSA POPULATION UNDER THE INFLUENCE OF CHEMICAL FACTORS IN THE HABITAT
EKOLOGIA POLSKA 35: 559-590 (1987)
Ryszard KAMIŃSKI
Botanic Garden, University of Wrocław, Sienkiewicza 23, 50-335 Wrocław, Poland
ABSTRACT: Ten habitats of A. vesiculosa - disappearing in Europe aquatic plant, have been investigated. The habitats were characterized according to water chemistry, composition of bottom sediments, describing also the coenotic relations, i.e., biotic ones in the phytosociological aspect. An analysis of individual and group characters of plants allowed to find chemical factors of habitats most significant for the growth of plants examined, the best habitats for A. vesiculosa population were indicated, also water chemistry necessary for maintenance cultures or introductions was determined.
KEY WORDS: Aldrovanda vesiculosa, ecological differentiation of population, chemistry of habitats, humic acids, coenotic relations, introduction.
INTRODUCTION
Changes in natural environment causing the death of many plant species make it absolutely necessary to study more intensely their ecology and to find methods for their protection. Legal protection of endangered species is no longer sufficient. Active protection requires an interference in the fate of each endangered population of the disappearing species, and the least we can do is to find a substitute place. Therefore, it is indispensable to know the best physico-chemical and biotic conditions in the habitat.
At present the most endangered are water and marsh-plants as each interference of man frequently causes irreversible changes of aquatic relations in the habitat and of water chemistry. One of such plants is Aldrovanda vesiculosa - small, free floating, belonging to the family Droseraceae and thus carnivorous. Another interesting thing is that it cannot grow in a purely mineral range (A s h i d a 1934, 1935, S c u 1 t h o r p e 1971), but requires the presence of organic matter or accompanying plants. Its range of occurrence is rather wide (Europe, Asia, Africa, Australia), although the number of its localities is relatively small. The majority of them have been in Europe, but are no longer confirmed (acc. to IUCN data).

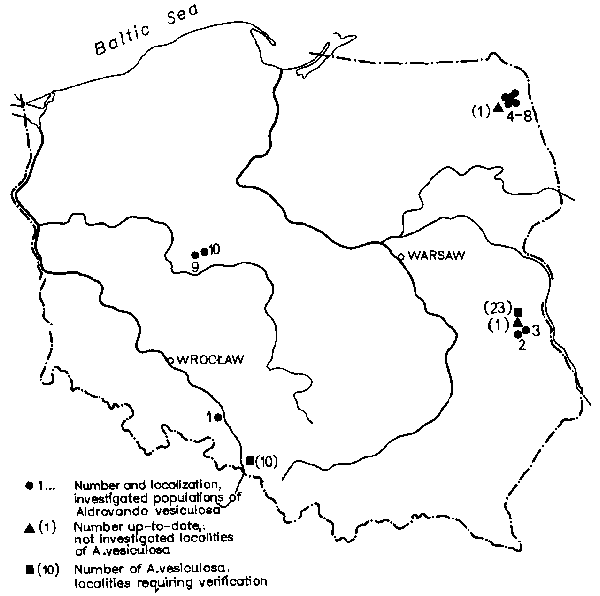
Fig. 1. Distribution of localities of Aldrovanda vesiculosa and those that should be verified in Poland
As the highest number of localities of A. vesiculosa in Europe was recorded from Poland I tried to determine the resources of this species in Poland. The exploration conducted between 1979 and 1981 covered 35 localities out of 74 known ones as the probability of finding the plant there was the greatest. The occurrence of A. vesiculosa was confirmed only on 12 localities (K a m i ń s k i 1983). Autecological investigations on 10 localities (Fig. 1) were conducted to provide answers:
(1 ) What is the ecological amplitude of A. vesiculosa, i.e., to what extent its habitats are differentiated, especially as regards water chemistry?
(2) Are the group and individual characters of natural A. vesiculosa populations differentiated and to what extent it is conditioned by the chemistry of habitat? Thus, it would be known what kind of habitat is the most suitable for this plant, and what are the worst factors affecting the plant populations causing their disappearance from particular localities.
METHODS OF INVESTIGATIONS
2.1. PHYTOSOCIOLOGICAL ANALYSIS OF A. VESICULOSA LOCALITIES
The phytosociological analysis, based on the system of M a t u s z k i e w i c z (1982) for precise definition of plant associations, among which A. vesiculosa occurs, included a comparison of 20 geobotanical records made by the Braun-Blanquet method (Matuszkiewicz 1967). Their area varied, depending on the area occupied by the population of the plant examined. They were in patches of communities with the highest density of populations examined.
2.2. CHEMICAL ANALYSIS OF WATER AND BOTTOM SEDIMENTS
Each microhabitat of Aldrovanda on localities examined was described according to the chemical characteristics of water and bottom sediments. Three samples of water from each microhabitat were taken at the depth of 5 - 20 cm at the turn of July in 1980 and 1981. Water pH was determined directly on localities. For nitrogen determination water was preserved with 3 ml CHC13.1-1 (G o m ó ł k a and S z y p o w s k i 1975) and for iron - with muriatic acid, 12 ml.l-1 of water (H e r m a n o w i c z et al. 1967). Before the analyses all samples were stored at 2°C.
The contents of nitrates, ammonia (using Nessler's reagent), phosphorus in the form of orthophosphates and iron were determined by the colorimetric method in water. The flame photometer (Johanson-Ulrich method) was used to determine the contents of potassium and natrium, EDTA method - for calcium and magnesium, nephelometric method - for sulphates and titration for chlorides. These methods are given by Hermanowicz et al. (1967), Lurie (1973), Gomółka and Szypowski (1975).
Total contents of organic substances, expressed by carbon content in water volume, were determined by a simplified method of Kononova and Belčikova. Carbon was determined in dry residue after the evaporation of previously filtered water in water bath at 60°C. The contents of free and connected with free-forms R2O3, i.e., not connected with calcium, of humic and fulvic acids were determined by Tjurin's method. Both methods are described by K o n o n o v a (1968).
In bottom sediments, presenting very well the trophic state of habitats, the contents of organic matter, calcium carbonate and silicates were determined by gravimetric method (L i t y ń s k i et al. 1972).
2.3. CHEMICAL ANALYSES OF PLANTS
Plants were sampled together with water from places varying as to the species composition of plants accompanying the Aldrovanda. Plants were incinerated with perhydrol and sulphuric acid. Then a water solution was made, in which nitrogen was determined by the turbidimetric method (N o w o s i e 1 s k i 1968), phosphorus by the colorimetric method with molybdate (B a b k o and P i 1 i p i e n k o 1955), potassium and sodium - in a flame photometer using the Johnson-Ulrich method, calcium and magnesium - by the EDTA method (B a r r o u s and S i m p s o n 1962) and iron - by the colońmetric method with alpha-alphabipyridyle according to P i p e r (1957). Sulphur was determined by the Butters-Chenry method (N o w o s i e 1 s k i 1968), whereas the ash content in dry plant weight - by combustion in a muffle furnace (600°C, 6 hours). All measurements were repeated three times.
2.4. SELECTION OF INDIVIDUAL AND GROUP CHARACTERS OF PLANTS AND THEIR MEASUREMENTS
The experimental areas were varying as to the species composition of accompanying plants. The size of experimental areas was 0.25 - 1.0 m2, depending on the density of plants, 30 plants of A. vesiculosa being the minimum (with the exception of locality 1 at Nowa Kuźnia, where the experimental areas covered more than 200 m2). In the biometrical analysis each population was represented by three samples, i.e., 90 plants. The biometrical analysis included: plant length, number of whorls, mean length of internodes, number of leaves in a whorl (mean number of leaves in three middle whorls), length of leaves (mean of 3 measurements in middle whorls), number of setae (mean from visual inspection of 5 optional leaves), number of branches, their mean length and number of buds in leaf whorls. The measurements are given in millimetres.
As regards the group characters, i.e., the population ones of A. vesiculosa, analysed were: plant density, i.e., number of individuals per 1 m2, biomass of 100 plants in mg dry weight as an indicator of habitat productivity in relation to Aldrovanda and the biotic population potential. The index of biotic potential was assumed as the ratio of the sum of buds, branches and young individuals to the number of plants in the sample. As young plants were accepted plants without the brown tints on leaves in the oldest whorls, so separated not long age from parent plants.
2.5. EXPERIMENTS IN CULTURES IN VITRO
Indispensable experiments in cultures in vitro were conducted to determine the effect of basic chemicals in the habitat on the growth of plants examined. Examined were apical plant segments, 30 mm long, from Lake Kruglak (experiment 1) and the pond at Nowa Kuźnia (experiment 2).
E x p e r i m e n t 1. It followed a series of preliminary experiments to determine the best medium composition and was conducted in two parts (A and B). The aim was to determine the effect of more important macroelements (ammonium and nitrate forms of nitrogen, phosphorus, potassium, magnesium and humic acids in the form of sodic humiane including the joint effect of humic forms and mineral compounds) on the growth of plants examined. In experiment A conducted between June 12 and July 7, 1981, a modified medium of Hampe and Truffaut was used (B u c z e k et al. 1976) with an addition of microelements acc. to H e 1 1 e r (1953) and sodic humiane. The basic medium contained (in mg.l-1): NO3 - 0.62, NH4 - 0.54, PO43- - 0.47, K+ - 3.12, Mg2+ - 2.92, Na+ - 4.83, SO42- - 16.23, Cl- - 21.20, Ca2+ - 8.02, Fe2+ - 0.55 in the form of Fe EDTA, and microelements - 1 ml of Heller solution, and also C of organic matter - 5000 microg. Initial pH 6.5, the changes not exceeding ± 0.3 at the end of experiments. The experiment was repeated three times with 10 plants in a crystallizer as a sample. The medium was protected against evaporation by a synthetic film (Parafilm), permeable for gases. Medium temperature was 18°C, at diffuse day light. When changing the concentrations of factors examined, other remained at a level resembling the basic medium. The experiment terminated when the plant growth showed signs of regression.
As the results of experiment A were not explicit, the effect of ammonia, nitrates and phosphorus was examined again (experiment B) between June 18 and July 14, 1984. For faster and longer time of plant growth a germinant rhizome of Carex rostrata was placed in each crystallizer. Other factors were the same as in part A of the experiment.
E x p e r i m e n t 2. Conducted between June 6 and September 9, 1980, it aimed at determining the ecological optimum of A. vesiculosa in relation to water pH. In each aquarium with unfiltered water from natural habitat and rhizomes of Typha latifolia, 5 Aldrovanda plants were placed. Water acidity (pH) was adjusted by 2.5 n NaOH and 2 n HCl within 2.5-3.5, 3.5-4.5, 4.5-5.5, 5.5-6.5, 6.5-7.5, 7.5-8.5, 8.5-9.5. Because of insufficient plant material there were only two replicas of the experiment.
2.6. STATISTICAL AND MATHEMATICAL METHODS
The statistical methods used in elaborating the material were: analysis of variance, two-variable and multiple correlation. Analysis of variance with two factors according to the constant model (E 1 a n d t 1964) was used to estimate the variability of microhabitats and A. vesiculosa populations, the least significant difference (LSD) and also for testing the results of experiments. Tables with characteristics of microhabitats, habitats and populations give the importance of variance differentiation (IVD) determined by F-Snedocor test at probability level 0.05 (*), 0.01 (**) and 0.001 (***). For comparison of F values statistical tables were used (Z i e 1 i ń s k i 1972). Statistical size indices, allowing to arrange habitats and A. vesiculosa populations (Fig. 2), were calculated by means of standard characters. Two-variable and multiple correlations between particular plant and habitat characters were calculated in biologically justified cases. R a o (1965) described methods for determining coefficients of two-variable and multiple correlations and for testing the significance.
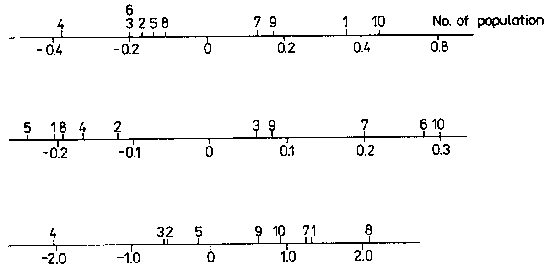
Fig. 2. Statistical quantities - classification according to standard characters. a - classification of habitats acc. to total chemistry of water, b - Aldrovanda vesiculosa population arranged acc. to individual and group characters, c - A. vesiculosa population arranged acc. to nutrient content in plants.
RESULTS
DESCRIPTION OF HABITATS WITH CONSIDERATION TO COENOTIC RELATIONS
The habitat of population examined is:
(1) The southern part of overgrown pond formed by impounding the Proszkówka river at Nowa Kuźnia near Opole.
(2) North-western coastal waters of the mid-forest, dystrophic Lake Długie of Łęczna-Włodawa Lake District.
(3) North-eastern coastal waters on peat of the dystrophic lake Brzeziczno (Łęczna-Włodawa Lake District), where as in Lake Długie the bottom is covered by a thick layer of organic silt and is poorly overgrown by plants.
(4) Depression with water in a turf cover on one of the turning into peat bays of Lake Krzywe in the Augustowska Forest (at the outlet of Augustowski Channel to Lake Miklaszewo).
(5) Meander of the channel between Lake Krzywe and Kruglak.
(6) Eastern coastal zone adjoining a large community of the class Scheuchzerio-Caricetea fuscae of the dystrophic Lake Kruglak.
(7) Eastern coastal waters of eutrophic Lake Miklaszówek near the outlet of Augustowski Channel turning into peat but with dense vegetation.
(8) Small bay on Lake Krzywe close to the lock on Augustowski Channel.
(9) Small pool on a turning into peat valley of watercourse joining Lake Budziszewskie with Lake Powidzkie (some 200 m from the shores of the latter) of the Gniezno Lake District.
(10) Pool on the turning into peat northern shore of the dystrophic Lake Salomonowskie of the Gniezno Lake District.
The common thing for all these habitats are shallow eutrophic and dystrophic lakes, especially peaty, shallow (1 m deep), sheltered from wind and well heating coastal parts of these lakes. The bottom is covered by a thick layer of mineral-organic or organic silt. Underwater vegetation is much differentiated there, i.e., from very poor (localities 2, 3, 6) to abundant (locality 7). Usually A. vesiculosa grows in a narrow belt of nympheides and sedge rushes (sporadically in bulrushes) adjoining the forming communities of the class Scheuchzerio-Caricetea fuscae. It occurs less frequently in depressions of the moss-sedge turf overgrowing the lake. The analysis of phytosociological records (Table 1) shows that Aldrovanda occurs in different floristic associations, thus having a broad phytosociological amplitude. Usually its occurrence within one habitat has a mosaic character. In habitats examined it is a component of plant communities belonging to two phytosociological classes - Potamogetonetea and Phragmitetea - where it grows better. Among plant associations, especially those from the alliance Magnocaricion, it has the highest frequency and density. A. vesiculosa is most frequently accompanied by Hydrocharis morsus-ranae, Stratiotes aloides, various species of Carex, Typha latifolia and Utricularia vulgaris.
Table 1. Plant aggregates with Aldrovanda vesiculosa (see Table 1 on separate page)
3.2. CHEMISTRY OF WATER AND BOTTOM SEDIMENTS
Table 2 gives chemical characteristics of water of Aldrovanda habitats examined in 1980, which allowed to present statistically the size of habitats from the point of total water chemistry (Fig. 2) dividing them into extreme habitats (4, 10), intermediate habitats (6, 7) for further investigations.
Water from habitats examined was slightly acid (pH from 5.57 on locality 3 to 6.8 on locality 6) and soft. The water abundance in food components was determined according to P a t a 1 a s (1960a, 1960b) system. Aldrovanda occurs in habitats most resembling the lakes from the first group in this classification, i.e., shallow ones without permanent thermal stratification in summer. Thus the water of habitats examined with the exception of the first locality had low nitrate content (0.10-0.37 mg.l-1). Ammonium nitrogen content was high on the average and significantly differentiated. It was the lowest (0.5 - 1.03 mg.l-1) in habitats with flowing water (5, 6, 8) and the highest (3.08 mg.l-1), where organic substance accumulation was faster than its mineralization, i.e., on localities 3, 7, 9, 10, or when there was a possibility of runoff of ammonium salts from fields and of liquid manure from neighbouring farm buildings (localities 1, 9, 10). Generally, the habitats do not differ as to phosphate content in water, which during the period of greatest development of aquatic vegetation was high, on the average 0.03-0.085 mg.l-1. The majority of habitats, except habitats 1 and 10, did not differ as to potassium content in water. They were averagely abundant in this element (1.23 - 7.30 mg.l-1). Habitats with the highest concentration of ammonium nitrogen in water (1, 7, 10) were the most abundant in potassium. But in the habitats examined the contents of calcium were low in habitat 3 (7.77 mg.l-1), averagely low in habitats 1 and 2, average in habitats 4, 5, 6, 7, 8 and high in habitats 9 and 10 (85.6 mg.l-1); of iron - were the lowest in habitats 6 and 8 (0.05 mg.l-1) and the highest in habitat 1 (1.44 mg.l-1), and of chlorides were also differentiated. Magnesium content was relatively low, from 1.73 mg.l-1 in habitat 1 to 15.03 mg.l-1 in habitat 10. Its low content in relation to calcium indicates poor mineralization in these habitats (G o m ó ł k a and S z y p o w s k i 1975). The sodium content was also low and less differentiated, from 3.6 mg.l-1 in habitat 4 to 16.2 mg.l-1 in habitat 10. The sulphate content was also low and not much differentiated, being close to the average in the majority of habitats, i.e., 21.52 mg.l-1.
Table 2. Characteristics of habitats of Aldrovanda vesiculosa populations
|
No. of popula- tions |
Day of sampling |
pH |
Contents (mg.l-1 of water) |
Carbon-ate hardness |
Contents of organic substances (microg C.l-1) |
||||||||||
|
N-NO3 |
N-NH4 |
P-PO4 |
K |
Ca |
Mg |
Na |
Fe |
SO4 |
Cl |
total carbon |
carbon of humic acids |
||||
|
1 |
1980.07.22 |
6.57 |
1.63 |
2.08 |
0.076 |
5.63 |
23.91 |
2.16 |
16.17 |
1.44 |
41.49 |
17.90 |
3.56 |
2917 |
2726 |
|
2 |
1980.08.01 |
6.43 |
0.23 |
1.45 |
0.085 |
2.23 |
19.23 |
1.73 |
6.01 |
0.51 |
21.69 |
7.73 |
2.91 |
3647 |
3260 |
|
3 |
1980.08.03 |
5.57 |
0.23 |
2.11 |
0.045 |
4.77 |
7.77 |
2.43 |
7.58 |
0.13 |
10.11 |
7.77 |
1.62 |
6028 |
5791 |
|
4 |
1980.08.08 |
6.30 |
0.12 |
1.11 |
0.049 |
1.77 |
44.03 |
2.27 |
9.78 |
0.10 |
10.21 |
10.03 |
6.72 |
1872 |
1335 |
|
5 |
1980.08.08 |
6.63 |
0.17 |
0.50 |
0.065 |
1.77 |
47.86 |
2.42 |
9.35 |
0.06 |
21.51 |
11.33 |
7.16 |
3642 |
3464 |
|
6 |
1980.08.08 |
6.37 |
0.07 |
1.03 |
0.068 |
1.43 |
42.22 |
3.02 |
6.94 |
0.05 |
28.37 |
6.00 |
6.55 |
3878 |
3848 |
|
7 |
1980.08.09 |
6.03 |
0.23 |
2.05 |
0.067 |
3.34 |
63.63 |
6.00 |
10.00 |
1.31 |
28.33 |
6.00 |
10.17 |
3736 |
3577 |
|
8 |
1980.08.09 |
6.47 |
0.10 |
0.90 |
0.074 |
2.87 |
48.52 |
4.72 |
8.99 |
0.05 |
21.33 |
2.07 |
7.84 |
3701 |
3660 |
|
9 |
1980.08.11 |
6.53 |
0.37 |
1.30 |
0.065 |
2.23 |
67.40 |
5.11 |
11.51 |
0.22 |
16.08 |
13.87 |
10.53 |
6326 |
4753 |
|
10 |
1980.08.11 |
6.33 |
0.37 |
1.38 |
0.070 |
4.23 |
85.62 |
15.03 |
15.76 |
0.26 |
16.00 |
14.03 |
15.40 |
6233 |
5078 |
|
Total mean |
6.32 |
0.35 |
1.39 |
0.070 |
3.04 |
45.02 |
4.49 |
10.21 |
0.4) |
21.51 |
9.67 |
7.25 |
4198 |
3779 |
|
|
Least significant difference (LSD) |
0.12 |
0.41 |
0.14 |
- |
2.43 |
0.75 |
0.46 |
3.43 |
0.19 |
2.87 |
0.42 |
0.16 |
1182 |
1831 |
|
|
Fcalculated (Ftab. 2.39) |
59.4 |
46.7 |
168.5 |
0.80 |
2.99 |
8481 |
621 |
6.81 |
66.8 |
94.67 |
1084 |
4845 |
13.9 |
3.68 |
|
|
Importance of variance differentiation (IVD) |
*** |
*** |
*** |
- |
* |
*** |
*** |
*** |
*** |
*** |
*** |
*** |
*** |
** |
|
|
4 |
1981.07.29 |
6.70 |
0.13 |
0.69 |
0.047 |
1.99 |
44.28 |
8.57 |
3.60 |
0.41 |
20.59 |
13.62 |
7.57 |
2526 |
1727 |
|
6 |
1981.07.29 |
6.80 |
0.05 |
0.49 |
0.030 |
1.23 |
27.62 |
7.01 |
4.91 |
0.25 |
27.95 |
7.42 |
5.73 |
1803 |
1140 |
|
7 |
1981.07.29 |
6.70 |
0.17 |
1.26 |
0.044 |
1.40 |
44.84 |
8.57 |
3.74 |
0.26 |
21.36 |
4.87 |
7.56 |
1904 |
1266 |
|
10 |
1981.07.30 |
6.60 |
0.18 |
3.08 |
0.082 |
7.30 |
53.34 |
6.57 |
16.20 |
0.45 |
16.18 |
13.09 |
9.75 |
8957 |
5577 |
|
Total mean |
6.70 |
0.16 |
1.38 |
0.052 |
2.98 |
42.52 |
7.68 |
7.11 |
0.34 |
21.52 |
9.75 |
7.65 |
3798 |
2427 |
|
|
Least significant difference (LSD) |
|
- |
0.71 |
0.015 |
0.52 |
5.86 |
- |
1.09 |
0.12 |
3.34 |
1.31 |
0.65 |
672 |
746 |
|
|
Fcalculated (Ftab. 4.07) |
|
0.84 |
29.7 |
23.33 |
35.9 |
112.8 |
0.99 |
334.4 |
7.23 |
22.45 |
22.8 |
39.5 |
280.9 |
85.5 |
|
|
Importance of variance differentiation (IVD) |
|
_ |
*** |
*** |
*** |
*** |
_ |
*** |
** |
*** |
*** |
*** |
*** |
*** |
|
The contents of organic substances were more differentiated in the water of habitats examined, the main component being the fulvic and humic acids soluble in water. The latter are high-molecular organic acids, dissociating poorly with pH of about 3.5. They are products of microbiological decomposition of organic substances, especially under anaerobic conditions. Water from habitats 9, 10 and 3 with carbon content over 6200 microg.l-1 was the most abundant in organic substances and thus in humic acids. Water from habitats 4 and 1 with organic carbon content 1872 and 2917 microg.l-1, respectively, was the least abundant in organic substances. In other habitats carbon content in water was 3600- 3900 microg.l-1. The contents of organic substances, similarly as of humic acids in them did not exceed the extreme values recorded by Semenov for lakes in the USSR (Starmach et al. 1978). Generally, it can be said that habitat 1 was the most abundant in mineral food components, then habitats 10 and 7 (Fig. 2), whereas the least abundant was habitat 4, from which not much different were habitats 3, 6, 5, 8 and 2.
The selected habitats were investigated in the following year, i.e., in 1982, and the results are presented in Table 2. These habitats differed significantly in the contents of nitrates and magnesium. Differences were also observed in relation to the preceding year, mostly concerning the contents of ammonium ions, calcium and to a smaller extent for sodium and organic compounds. Frequently the contents of macroelements exceeded the minimum (phosphorus, potassium, sodium in habitats 6, 7) and the maximum (ammonium, potassium and organic compounds in habitat 10) recorded in 1980. The least abundant habitat 4 was the most stable as regards the contents of inorganic substances, but the amount of organic substances in water increased in this habitat. The majority of data proves the relatively high variability of water chemistry in habitats examined, i.e., in shallow waters with abundant vegetation and mainly with a small water flow.
Table 3. Organic matter and calcium contents in bottom sediments of Aldrovanda vesiculosa habitats
|
No. of popula- tions |
Habitats |
Organic matter |
CaCO3 % |
Silicate (+ others) |
|
1 |
Pond "Staw Nowokuźnicki" |
15.34 |
0.47 |
84.19 |
|
2 |
Lake Długie |
76.40 |
0.69 |
22.91 |
|
3 |
Lake Brzeziczno |
74.14 |
0.90 |
24.96 |
|
4 |
Lake Krzywe |
72.64 |
0.82 |
26.44 |
|
5 |
Channel between Lake Krzywe and Kruglak |
78.67 |
2.06 |
19.27 |
|
6 |
Lake Kruglak |
83.40 |
0.89 |
15.71 |
|
7 |
Lake Miklaszówek |
22.07 |
10.29 |
66.64 |
|
8 |
Lake Krzywe (near sluice) |
28.26 |
0.78 |
70.96 |
|
9 |
Lake Powidzkie |
61.12 |
0.64 |
38.24 |
|
10 |
Lake Salomonowskie |
52.69 |
0.80 |
46.45 |
Knowing the contents of most important components of bottom sediments of Aldrovanda habitats allows to determine the type of sediments, and indirectly the trophic state of these habitats (Table 3). Attention should be called to the low calcium content in sediments examined which did not exceed 10.3% of dry sediments and a high percentage of organic matter, 53 – 83% dry wt. of sediments. Organic matter content in habitats 1, 7 and 8 was low, 15.34, 22.07 and 28.26%, respectively. Their eutrophic character is determined by the prevalence of silicates at low or average calcium content, whereas high content of organic sediments and low calcium content in habitats 2, 3, 4, 5, 6, 9 and 10 puts them within eutrophic-dystrophic or dystrophic habitats (acc. to Rybak after Starmach et al. 1978).
3.3. INDIVIDUAL AND GROUP CHARACTERS OF PLANTS
Individual and group characters of A. vesiculosa in 1980 and 1981 are given in Table 4. Populations differed as to their individual and group characters, the exception being the population density. The most important individual character analysed was the plant length. It was differentiated: the shortest plants were in habitat 1 (57.8 mm) and 8 (59.7 mm) and the longest - in habitat 10 (111.5 mm), 7 (107.1 mm) and 3 (106.9 mm). The smallest plant was 14.5 mm long (population 8), whereas the longest one - 181 mm (population 10). The differentiated number of leaf whorls also depended greatly on plant length. Plants from population 1 had the smallest number of leaf whorls (11.8), and those from population 3 had the highest number (18.7) although the average plant length was not the highest. Average distance between whorls was from 4.9 mm in population 1 to 7.1 mm in population 10, at an average of 5.5 mm. It should be pointed out that the distance between leaf whorls was directly proportional to the rate of plant growth.
The number of leaves in whorls was also significantly differentiated from 7.36 in population 10 to 8.44 in population 6, at an average 7.85. This character varied greatly. Within one plant there was sometimes a difference of 3 leaves in a whorl. The minimal number of leaves in a whorl was 5 and the maximal one 10. The number of setae on a leaf also greatly varied within one plant. There could be a difference of 3 setae, whereas within all populations the number of setae was 2 - 7. Plants from population 3 had the smallest number of setae (4.04), whereas those from population 9 had the highest number (4.44). Due to this great variability within plants populations did not differ significantly, the only exception being population 9. The most constant was the plant length, which affects significantly the population differentiation. Plants from populations 6 and 7 had the longest leaves, 8.51 and 8.49 mm, respectively, whereas those from population 5 had the shortest ones (6.56 mm); average length was 7.53 mm.
The number of branches (offshoots), their length and number of buds varied within one population. This differentiated the population. Population 10 had the most branched plants with 1.53 lateral shoots on the average, whereas plants from populations 2 and 3 had the smallest number of branches (0.27). The average length of lateral shoots was from 15.96 mm in population 1 to 26.13 mm in population 7. The number of lateral buds was from 0.12 on one plant in populations 5 and 9 to 0.55 in population 7.
The population density of A. vesiculosa - a free floating plant greatly varies depending on physical conditions of the habitat and density of plants among which it occurs. In habitats examined its density was 0.12 plant m-2 in population 1 to 341.3 plants m-2 in population 6. The density never seems to be of greater significance for Aldrovanda plants as it is never such as to disturb its development, because it grows equally well when floating under the water surface and deeper. Measurements of density of A. vesiculosa population allowed to estimate its numbers. Populations 1, 4, 5, 8 were small, not exceeding 1000 plants, populations 2, 3, 9 and 10 were average, up to 5000 individuals, whereas remaining populations (6 and 7) were much more abundant.
Table 4. Characteristics of ecological populations of Aldrovanda vesiculosa. For explanation of symbols see Table 2.
|
No. of population |
Individual feature |
Group feature |
||||||||||
|
plant length (mm) |
No. of leaf whorls per one plant |
average distance between the whorls (mm) |
No. of leaves in whorl |
length of leaves (mm) |
No. of setas per one leaf |
No. of branches per one plant |
Average Length of branches (mm) |
No. of axillary buds per one plant |
density of plants on 1 m2 |
biomass of 100 plants (mg d.wt.) |
Index of biotic potential (%) |
|
|
1980 |
||||||||||||
|
1 |
57.83 |
11.80 |
4.87 |
7.43 |
7.33 |
4.15 |
0.80 |
15.96 |
0.23 |
0.12 |
1794 |
116.9 |
|
2 |
74.26 |
13.63 |
5.45 |
7.60 |
7.30 |
4.18 |
0.27 |
20.81 |
0.15 |
13.35 |
1378 |
93.0 |
|
3 |
106.92 |
18.73 |
5.71 |
7.93 |
7.14 |
4.04 |
0.27 |
25.43 |
0.33 |
18.66 |
1543 |
133.2 |
|
4 |
64.89 |
13.49 |
4.80 |
7.89 |
7.51 |
4.10 |
0.41 |
18.30 |
0.17 |
15.00 |
1965 |
81.9 |
|
5 |
62.53 |
14.08 |
4.52 |
8.08 |
6.56 |
4.08 |
0.31 |
19.21 |
0.12 |
8.43 |
1370 |
68.9 |
|
6 |
95.30 |
16.49 |
5.78 |
8.44 |
8.51 |
4.17 |
0.69 |
22.37 |
0.40 |
341.33 |
2664 |
130.6 |
|
7 |
107.08 |
16.70 |
6.41 |
8.39 |
8.49 |
4.08 |
0.71 |
26.13 |
0.40 |
11.67 |
2642 |
123.6 |
|
8 |
59.68 |
12.32 |
4.83 |
7.92 |
6.96 |
4.07 |
0.40 |
17.53 |
0.33 |
8.67 |
1340 |
114.7 |
|
9 |
97.27 |
16.33 |
5.93 |
7.44 |
7.71 |
4.44 |
0.85 |
25.46 |
0.12 |
15.00 |
2350 |
119.8 |
|
10 |
111.45 |
15.74 |
7.08 |
7.36 |
7.78 |
4.15 |
1.53 |
24.75 |
0.45 |
34.33 |
2473 |
239.4 |
|
Total mean |
83.72 |
14.93 |
5.53 |
7.85 |
7.53 |
4.15 |
0.63 |
21.60 |
0.27 |
46.66 |
1955 |
122.2 |
|
LSD |
14.72 |
1.52 |
0.73 |
0.38 |
0.47 |
0.16 |
0.46 |
6.78 |
0.21 |
- |
705 |
37.7 |
|
Fcalculated |
18.96 |
14.35 |
10.2 |
8.95 |
13.4 |
442.5 |
5.64 |
2.64 |
3.01 |
1.96 |
1453 |
13.1 |
|
Ftab. |
1.91 |
1.91 |
2.39 |
1.91 |
1.91 |
1.91 |
2.39 |
2.39 |
2.39 |
2.39 |
2.39 |
2.39 |
|
IVD |
*** |
*** |
*** |
*** |
*** |
*** |
** |
* |
* |
- |
*** |
*** |
|
1981 |
||||||||||||
|
4 |
79.58 |
16.30 |
4.77 |
8.22 |
8.24 |
4.35 |
0.93 |
19.18 |
0.50 |
43.33 |
2727 |
145.0 |
|
6 |
60.35 |
16.37 |
4.32 |
8.39 |
8.14 |
4.22 |
0.78 |
24.89 |
0.35 |
281.33 |
1960 |
113.3 |
|
7 |
85.17 |
15.76 |
5.30 |
8.26 |
8.41 |
4.40 |
0.97 |
24.37 |
0.55 |
81.89 |
2398 |
153.2 |
|
10 |
76.17 |
14.83 |
5.14 |
8.23 |
7.89 |
4.27 |
1.15 |
22.73 |
0.28 |
101.67 |
2294 |
143.3 |
|
Total mean |
75.31 |
15.22 |
4.88 |
8.28 |
8.17 |
4.31 |
0.96 |
22.79 |
0.41 |
127.56 |
2345 |
138.7 |
|
LSD |
7.41 |
1.10 |
0.73 |
0.12 |
0.24 |
0.14 |
- |
- |
- |
162.79 |
401 |
- |
|
Fcalculated |
16.04 |
12.31 |
4.08 |
5.77 |
15.2 |
3.53 |
2.06 |
2.77 |
2.47 |
4.58 |
9.80 |
1.63 |
|
Ftab. |
2.60 |
2.60 |
4.07 |
2.60 |
2.60 |
2.60 |
4.07 |
4.07 |
4.07 |
4.07 |
4.07 |
4.07 |
|
IVD |
*** |
*** |
* |
** |
*** |
* |
- |
- |
- |
* |
** |
- |
* Index of biotic potential - the ratio of sum of lateral shoots (branches), axillary buds and young plants (lateral shoots recently separated from parent plants) to the number of plants in the sample.
The index of biotic potential, indirectly showing the usefulness of habitats for the growth of A. vesiculosa, varies greatly within the population and in time. It is the strongest indicator of physico-chemical changes in habitat conditions. Knowing the biology of Aldrovanda, it could be expected that this index being close to 100% or below would be for a regression population, 120-150% for a rather stabilized population, whereas over 150% - for a progressive population. Although the present investigations did not last long they confirmed in principle these assumptions. This was proved by the index for stabilized populations (6 and 7), developing at the given moment (10 and 3), and in vanishing populations (5 and 4). In the latter no A. vesiculosa plants were found in 1981.
Statistical indices of size of populations examined in 1980 and arranged according to individual and group characters divide these populations into two groups (Fig. 2b). However, the position of particular populations on the axis is not very consistent with that of habitats arranged according to total water chemistry (Fig. 2a). This shows that there are also other factors affecting the growth of plants (K a m i ń s k i 1987).
Biometric measurements of A. vesiculosa in 1981 showed that, similarly as in the previous year, some characters of plants indicated well the population differentiation. These were: plant length, number of leaf whorls, number of leaves in a whorl, their length and plant biomass. As regards remaining characters the populations either did not differ among themselves or differed only slightly. This was confirmed by results obtained in 1980 indicating a high intrapopulational variability of these characters. Also in relation to that year populations 10, 7 and 6 had generally smaller statistically differentiated individual and group characters, with the exception of density of populations 7 and 10 and the biotic potential of population 7. All characters of population 4 had higher values than in 1980.
3.4. FOOD COMPONENTS OF PLANTS
Table 5 gives the contents of food components in Aldrovanda vesiculosa and it can be said that the contents of macroelements in plants do not exceed the minimal and maximal (with the exception of phosphorus) values for other macrohydrophytes given, e.g., by Boyd (1968, 1970), Bernatowicz and Wolny (1974), Gaudet (1974), but approximate their contents in Utricularia sp. (Dykyjová 1979) of a similar biology and occupying a similar ecological niche. This concerns especially nitrogen, calcium, magnesium, sodium and sulphur.
Nitrogen content was 1.9% in plants from population 6 to 3.21% in plants from population 8. Its mean content approximated that in Utricularia sp. (2.5%), and the extreme values did not exceed the minimum and maximum for this species.
Phosphorus content was exceptionally high in A. vesiculosa, from 0.73% in plants from habitat 4 to 1.27% in plants from habitat 8. Mean phosphorus content in plants examined was five times higher than in Utricularia and twice higher than the maximal one in these plants - 0.57%. According to B o y d (1968) algae have such a high phosphorus content, but W o ż a k o w s k a - N a t k a n i e c (1976-80) has found even higher content in some Spirodela polyrrhiza populations (up to 1.4%).
Potassium content in Aldrovanda was lower than in Utricularia (2.68% on the average). Plants from locality 6 had the lowest potassium content (0.87%) and the highest one (2.13%) was in those from locality 3.
Calcium content in Aldrovanda was low and corresponded with that in Utricularia and emergent macrohydrophytes, which according to B o y d (1968) contain 1-3% of calcium, whereas the submerged plants have a much higher content (3 – 14%). In our case plants from population 3 had the lowest calcium content (1.2%), whereas plants from population 8 had the highest one (2.47%).
Magnesium content approximated also that in emergent and not submerged plants, which had a higher content. Plants from population 8 had the lowest magnesium content (0.34%), whereas those from population 1 had the highest content (1.09%).
Sodium content was 0.35% in plants from population 1 to 1.09% in those from population 6.
Sulphur content also did not differ from that in other macrohydrophytes and Utricularia, being the highest in plants from population 1 (0.59%) and the lowest in those from population 7 (0.18%).
Aldrovanda contained relatively little iron as compared with Utricularia. Its smallest content was in plants from habitat 6 (0.25%) and the highest one in plants from habitat 1 (1.30%).
Ash contents in plants was very low: 12.1 - 17.6%. Plants with floating leaves have such low ash contents, whereas underwater plants have 15 - 30% of ash similarly as plants of the genus Utricularia, where ash is 27 – 30% of dry plant weight.
Table 5. Contents of macroelements in plants of Aldrovanda vesiculosa (mg. 100 g-1 d. wt.). For explanation of symbols see Table 2.
|
No. of population |
N |
P |
K |
Ca |
Mg |
Na |
S |
Fe |
Ash contents |
|
1980 |
|||||||||
|
1 |
2660 |
912 |
1207 |
1132 |
1095 |
345 |
590 |
1303 |
17.6 |
|
2 |
3173 |
1012 |
1583 |
1269 |
583 |
617 |
265 |
528 |
14.4 |
|
3 |
3032 |
1062 |
2127 |
1119 |
1094 |
388 |
217 |
253 |
13.2 |
|
4 |
2760 |
1059 |
1363 |
1686 |
718 |
860 |
298 |
257 |
12.9 |
|
5 |
2820 |
1104 |
1500 |
2355 |
613 |
680 |
307 |
267 |
13.2 |
|
6 |
2270 |
824 |
1033 |
1737 |
768 |
1093 |
169 |
253 |
13.6 |
|
7 |
2857 |
1220 |
1427 |
1166 |
1077 |
770 |
177 |
972 |
15.5 |
|
8 |
3213 |
1269 |
1783 |
2474 |
344 |
735 |
300 |
282 |
15.6 |
|
9 |
2510 |
1047 |
1330 |
1883 |
1011 |
1090 |
298 |
830 |
12.8 |
|
10 |
2663 |
1250 |
1683 |
1966 |
662 |
917 |
421 |
283 |
12.3 |
|
Total mean |
2796 |
1076 |
1504 |
1677 |
797 |
732 |
304 |
523 |
13.9 |
|
LSD |
212 |
- |
353 |
269 |
140 |
52 |
33 |
84 |
1.2 |
|
Fcalculated (Ftab. = 2.39) |
16.5 |
0.9 |
6.7 |
30.0 |
30.1 |
211 |
127 |
179 |
22.3 |
|
IVD |
*** |
- |
*** |
*** |
*** |
*** |
*** |
*** |
*** |
|
1981 |
|||||||||
|
4 |
1900 |
731 |
1133 |
1897 |
777 |
703 |
314 |
331 |
13.1 |
|
6 |
2152 |
1006 |
873 |
729 |
763 |
764 |
284 |
344 |
12.6 |
|
7 |
2349 |
956 |
914 |
1002 |
921 |
459 |
301 |
374 |
12.9 |
|
10 |
2093 |
849 |
1552 |
1375 |
709 |
717 |
292 |
281 |
12.1 |
|
Total mean |
2124 |
885 |
1113 |
1251 |
790 |
660 |
298 |
333 |
12.7 |
|
LSD |
- |
193 |
200 |
276 |
83 |
125 |
- |
- |
1.05 |
|
Fcalculated (Ftab. = 4.07) |
2.04 |
4.25 |
25.7 |
38.6 |
14.2 |
128 |
1.5 |
1.0 |
5.3 |
|
IVD |
- |
* |
*** |
*** |
** |
** |
- |
- |
* |
A. vesiculosa populations examined in 1980 were significantly differentiated in the contents of elements, except phosphorus, whereas in the following year the range of differentiation was smaller. This may be due to less data, decreasing thus the statistical confidence interval, or to some other reasons (see Discussion). It is quite possible that at differentiated contents of particular macroelements in habitats the plants in relation to the previous year contained less calcium, potassium and nitrogen by some 26%, 18% less phosphorus and 9% less sodium. Their ash contents were also 9% lower.
3.5. POPULATION CHARACTERISTICS AND HABITAT CONDITIONS
The effect of some chemical factors of the habitat on the growth of Aldrovanda vesiculosa is shown by simple relations in Table 6.
The length of A. vesiculosa shoots is positively correlated with organic matter content in water and especially that of humic and fulvic acids. The more acids in the habitat the bigger the plants. The longest plants were in habitats 10 and 3 with high humic acid content in water and acid reaction, whereas in habitats 4 and 1 with a low humic acid content the plants had short shoots. Plant length is greatly conditioned by the water reaction expressed by negative correlation. The more acid the water the longer the shoots of plants examined. This is also confirmed by the multiple correlation (Table 7). Under natural conditions pH decreases with the increasing humic acid content, so these two factors act together. These relations have been checked experimentally. Results of experiment 1 (Table 8) show that the positive relation between plant growth and humic acid content (at constant water reaction) is only up to humic acid content about 6000 - 7000 microg C.l-1 of water. Further increase has an unfavourable effect on plant growth.
Results of the next experiment (Fig. 3) show that Aldrovanda is a species having a broad ecological scale in relation to pH of the habitat. Still, the biggest plants growing the quickest (the length of main shoot and lateral shoots together) and having the greatest number of lateral shoots produced by one parent plant, tantamount to biotic potential, grew at pH 4. In a more acid habitat (pH less than 3.5) the plants died, whereas pH increase limited the growth rate of plants examined. The poorest growth was recorded at pH 9. At water acidity pH 5.5- 7.5, such as under natural conditions, differences in plant length resembled those in natural habitats described by the regression equation.
There is also a positive correlation between iron content in plants and its content in water; the more abundant water in iron the more of this element in plants. At level of significance α > 0.05 potassium, calcium and sulphur contents depend positively on the amount of these macroelements in water, and the plant length - on sodium and magnesium contents.
Phosphorus and nitrogen contents increase in plants affect negatively their length, and although confirmed by multiple correlations it is not a typical tendency. Nevertheless, these relations are worth mentioning as they were observed in two successive years of investigations.
Complex effect of ecological factors, i.e., water chemistry and nutrient contents in plants, on A. vesiculosa development is best shown by multiple correlations (Table 7).
This concerns such individual characters as plant shapeliness and group characters, which allow for broader estimation of particular habitats for populations examined.
A. vesiculosa shoots are the longer the higher the contents of organic substances and magnesium in water and thus in plants and the higher sodium and potassium contents in plants. The length of shoots decreases with the increasing water pH, sulphate content in water, calcium, nitrogen and phosphorus content in plant.
Table 6. Correlations between length of plants and water chemistry and between the contents of nutrients in water and in plants.
|
No. |
Relations |
Year |
Regression equation |
Correlation coefficients (R2) |
F test for R2 |
t-Student test |
Probability (α) |
|
1 |
Length of plants and contents of organic substances in water |
1980 |
y = 38.3 + 0.01 x |
0.55 |
9.78 |
3.13 |
0.01 |
|
2 |
Length of plants and pH |
1980 |
y = 353.1 + 42.6 x |
0.373 |
4.77 |
-2.18 |
0.05 |
|
3 |
Fe level in plants and in water |
1980 |
y = 255.7 + 648.3 x |
0.806 |
3.31 |
5.77 |
0.001 |
|
When α > 0.05 |
|||||||
|
4 |
K level in plants and in water |
1980 |
y = 1262.9 + 79.3 x |
0.133 |
1.23 |
1.11 |
0.30 |
|
1981 |
y = 755.1 + 113.6 x |
0.332 |
2.13 |
1.98 |
0.14 |
||
|
5 |
Ca level in plants and in water |
1980 |
y = 1193 + 10.8 x |
0.259 |
2.79 |
1.67 |
0.13 |
|
1981 |
y = 486.2 + 14.5 x |
0.224 |
1.73 |
1.31 |
0.24 |
||
|
6 |
S level in plants and in water |
1980 |
y = 188.1 + 4.40 x |
0.170 |
1.64 |
1.28 |
0.24 |
|
1981 |
y = 285.5 + 0.70 x |
0.265 |
1.98 |
0.69 |
0.21 |
||
Table 7. Multiple correlations between: A - individual and group characters of Aldrovanda and general habitat chemistry (pH, content of organic substances and macroelements), B - individual and group characters of Aldrovanda and level of nutrients of Aldrovanda.
|
No. |
Relations |
Year |
Regression equation |
R2 |
Ftest. |
tStud. |
α |
|
A. 1 |
length of plants and pH, contents of organic substances, magnesium and sulphate in water |
1980 |
y = 38.3 + 0.01 xC - 42.02 xpH |
0.55 |
9.78 |
tC 3.1
tpH -3.0 |
0.014 |
|
1981 |
y = 87.7 + 5.71 xMg - 2.53 xS |
0.91 |
26.2 |
tMg 6.9 tS –4.8 |
0.002 |
||
|
2 |
No. of branches (offshoots) and pH, contents of nitrate, potassium and magnesium in water |
1980 |
y = 4.58 - 0.63 xpH + 1.03 xNO3 - 0.28 xK + 0.12 xMg |
0.98 |
54.9 |
tpH -3.5 tNO3 5.9 tK -4.9 tMg 12.6 |
0.0001 |
|
3 |
length of leaves and contents of potassium, magnesium. ammonium and iron in water |
1981 |
y = 7.22 + 0.44 xNH4 - 0.30 xK + 0.05 xMg + 2.56 xFe |
0.95 |
15.6 |
tNH4 5.6 tK -7.8 tMg 4.5 tFc 4.5 |
0.024 |
|
4 |
index of biotic potential and contents of calcium, magnesium, potassium, sodium and sulphate in water |
1980 |
y = 100.22 – 1.05 xCa + 15.37 xMg |
0.91 |
37.4 |
tCa -2.9 tMg 7.3 |
0.0002 |
|
1981 |
y = 328.1 – 22.11 xK + 6.39 xNa - 7.86 xS |
0.99 |
376 |
tK -24.2 tNa 23.4 tS –2.9 |
0.0001 |
||
|
5 |
plant biomass and contents of calcium, magnesium, iron and sulphate in water |
1981 |
y = 3113 - 33.7 xCa + 256.8 xMg + 2628 xFe - 97.7 xS |
0.98 |
41.9 |
tCa -4.9 tMg 10.4 tFe 5.2 tS -8.5 |
0.0001 |
|
B. 1 |
length of plants and contents of macro- elements in plants |
1980 |
y = 90.9 - 0.04 xN + 0.07 xK - 0.03 xCa + 0.08 xNa + 0.03 xMg |
0.93 |
16.0 |
tN -2.6 tK 5.5 tCa -5.0 tNa 4.8 tMg 1.8 |
0.005 |
|
1981 |
y = 552.3 - 0.04 xN - 0.45 xP - 0.08 xCa + 0.07 xNa |
0.99 |
826 |
tN -11.0 tP -44.2 tCa -43.9 tNa 21.2 |
0.001 |
||
|
2 |
No. of branches and contents of macro- elements in plants |
1980 |
y= -0.83 + 0.001 xNa + 0.002 xS |
0.70 |
5.21 |
tNa 2.7 tS 2.6 |
0.041 |
|
3 |
index of biotic potential and contents of macro- elements in plants |
1981 |
y = 107.4 - 0.08 xN + 0.21 xMg – 0.02 xCa |
0.71 |
6.11 |
tN -2.8 tMg 3.5 tCa -2.3 |
0.045 |
|
|
Total contents of organic substances and contents of mineral substances in water |
1981 |
y = 1076 - 19.41 xCa + 1048.7 xK - 86.1 xNa |
0.99 |
401 |
tCa -1.7 tK 33.7 tNa -5.8 |
0.001 |
Table 8. Influence of macroelements and humic acids on growth of cultured Aldrovanda vesiculosa (part A and B of experiment 1). For explanation of symbols see Table 2.
|
Part A of experiment |
Part B of experiment |
||||||
|
total contents of macroelements in relation to basis medium (C) |
length of plants (mm) |
Contents of humic acids (microg C.1-1)
|
length of plants (mm) |
Combinations of macroelements and humic acids |
length of plants (mm) |
contents of ammonium (mg.l-1)
|
length of plants (mm) |
|
C . 20 |
37.6 |
traces |
30.0 |
C . 20 + traces of humic acids |
31.2 |
0.12 |
85.0 |
|
C . 10 |
38.8 |
2000 |
39.4 |
C . 10 + 2000 |
40.6 |
0.36 |
111.1 |
|
C . 2 |
50.0 |
3600 |
40.6 |
C + 5000 |
46.4 |
0.62 |
87.8 |
|
C |
52.8 |
5000 |
47.3 |
C : 5 + 10000 |
46.6 |
1.86 |
75.5 |
|
C : 5 |
53.3 |
10000 |
39.3 |
C : 10 + 50000 |
41.2 |
3.62 |
74.9 |
|
C : 7.5 |
56.4 |
50000 |
36.7 |
C : 20 + 100000 |
41.0 |
|
|
|
C : 10 |
50.2 |
100000 |
33.8 |
C : 50 + 150000 |
32.0 |
|
|
|
C : 15 |
49.6 |
150000 |
29.1 |
|
|
|
|
|
C : 20 |
45.4 |
|
|
|
|
|
|
|
Fcalculated |
5.54 |
|
7.28 |
|
6.41 |
|
3.47 |
|
Ftab |
2.21 |
|
2.31 |
|
2.42 |
|
2.78 |
|
LSD |
7.52 |
|
5.80 |
|
5.98 |
|
24.00 |
Plant growth expressed by the number of lateral shoots is positively correlated with water acidity, nitrate and magnesium contents in water, sodium and sulphur contents in plants, and negatively correlated with potassium content in water.
Length of leaves is positively correlated with magnesium, iron and ammonia contents in water and negatively - with potassium content in water and nitrogen, sodium and calcium contents in the plant.
Biotic potential of A. vesiculosa population depends positively on magnesium and sodium contents in water, and magnesium content in plants, and negatively on calcium content in water and in plants, potassium and sulphate contents in water, and sodium content in plants.
Biomass of A. vesiculosa, expressing the productivity of habitat in relation to this plant, is positively correlated with magnesium and iron contents in water, and negatively - with calcium and sulphate contents in water.
However, this effect of some chemical factors under natural conditions may be due to other co-occurring factors or may not occur at all (e.g., negative effect of potassium). To dispel these doubts an experiment (No. 1) was made to determine the effect of phosphorus, potassium, magnesium, nitrates and ammonia and their optimum contents at constant contents of other macroelements and a lack of additional food source such as micro- and macroplankton. Unfortunately, the experiment was not successful. Perhaps the introduction of macroelements in amounts too high to have a limiting effect, together with an extract of humic acids, indispensable for the growth of A. vesiculosa, was responsible for that. This also concerns the effect of ammonium ions as the results obtained do not correspond well enough with results obtained when investigating natural habitats. However, it was confirmed that a not too high content of mineral salts in the medium, approximating that in natural habitats, is the optimum (Table 8). Higher or lower contents affect negatively the plant growth. Aldrovanda grows also the best when there is a certain equilibrium between the contents of inorganic and organic compounds in the habitat. When it is significantly disturbed the plants may die.
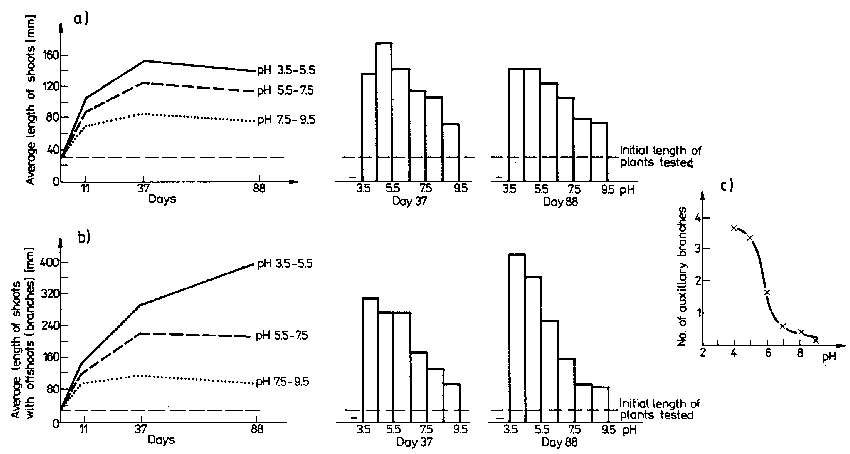
Fig. 3. The effect of water reaction on the growth of Aldrovanda vesiculosa in a culture in vitro. a - dynamics of main shoots' growth and diagrams of their length at different pH of water, b - dynamics of growth of main and lateral shoots and diagrams of their length at different pH of water, c - mean number of lateral shoots per plant in the final stage of experiment depending on pH of water.
DISCUSSION
Aldrovanda vesiculosa is a rare plant growing in different climatic zones. Its broad range indicates a great differentiation of physical (depending on climate) and biotic conditions of its habitats (Wendt 1952, H e g i 1961, S c u 1 t h o r p e 1971, S z a f e r 1972 and others). Chemical conditions are also differentiated. Thus it can be assumed that physico-chemical and biotic differentiation of A. vesiculosa habitats examined do not cover the ecological amplitude of the species.
In Poland the species was recorded from coastal waters of lakes of a different trophic state; oligotrophic, meso-, eu-, alloitrophic and dystrophic (Fijałkowski 1958a, 1958b, 1959, Michalak 1963, Żukowski 1963, Krawiecowa and Kuczyńska 1964, Panfil 1978, Sokołowski 1972). This classification, on the basis of entire physico-chemical and biotic conditions of the lake, not always shows the real trophic state of coastal lake waters, and especially those in little bays where Aldrovanda occurs. This is best proved by a similar ion content in waters of habitats 2, 3, 4, 5, 6, 8 and 9, i.e., from habitats determined as eutrophic, alloitrophic (where high content of inflowing calcareous salts prevents excessive acidification of habitat by humic substances) and dystrophic.
Although the chemistry of waters in which Aldrovanda occurs varies and is significantly differentiated, this variability is small and the ecological scale of A. vesiculosa compared with other macrohydrophytes is narrow (Wożakowska-Natkaniec 1976-1980, Stebnicka 1979, Słodczyk 1984a, 1984b). According to criteria of P a t a 1 a s (1960b) waters from habitats examined are not greatly abundant in nitrates, have an average potassium content, slightly more than the average of phosphates and ammonia and are relatively differentiated as regards calcium content. The contents of remaining macroelements are low (sulphates, chlorides, sodium, magnesium) with the exception of iron. The rather high iron content may prove the oxygen deficiency at the bottom, despite rather low water depth of habitats examined. In favour of this is the higher content of phosphates, which at pH 5 - 7 under anaerobic conditions are released easier into the environment and do not precipitate with iron in the form of ferric sulphate (B a c k m a n and B r a d y 1971 ), and also the high ratio of ammonia to nitrate ions in clean waters of habitats (except habitat 1 and possibly habitats 9 and 10).
Low content of chlorides, sodium and magnesium in water and high sapropel content in bottom sediments prove the poor mineralization of the habitat. This is also confirmed by multiple correlation (Table 7) between organic substance content (humic acids) in water and the calcium and sodium contents in water (negative correlation) and also potassium content (positive relation). This is consistent with generally known relations (Gomółka and Szypowski 1975, Starmach et al. 1978) that with the increasing dystrophy of habitats the contents of inorganic salts in water decrease with the exception of potassium, whereas the contents of humic acids increase. Humic acids and calcium have the greatest influence on differentiation of chemistry of habitats (Radwan et al. 1974).
All this, as well as the presence of some plant associations, allows to state that A. vesiculosa occurs first of all in habitats having a transitory character between eutrophic and dystrophic habitats. Populations there are most abundant and plants are shapely. A. vesiculosa occurs less frequently in dystrophic and typical eutrophic habitats, although sometimes it finds there suitable development conditions (habitat 7), but does not occur in oligo- and mesotrophic habitats.
Relations between habitat and population can be shown properly when chosing proper individual and group characters of population variability. Those with the highest ratio of the coefficient of interpopulation variability to that of intrapopulation one should be chosen as physico-chemical and biotic factors are less variable within one habitat change than in all habitats. Nevertheless, not all characters determine to the same extent the best habitat conditions. As pointed out by Rabotnov (1950) and U r a n o v (1960), ecological constitution of plants examined is expressed by the following characters: plant length, number of shoots, length of leaves, plant biomass and index of biotic population potential. They were used mainly in the present paper and should serve in the future to estimate and bioindicate deformations of A. vesiculosa habitat. In some cases the knowledge of mean distances between leaf whorls may be useful as it shows well the growth rate of plants.
Among chemical factors in the habitat, reaction has a visible effect on Aldrovanda. The general opinion is that Aldrovanda grows best at pH from 5.5 to 6.5. But acc. to Mazrimas (1974) the best pH is about 4.5. Field investigations and correlation equations indicate that at pH from 5.57 to 6.63 the plant length and the number of shoots and thus the index of biotic population potential are greater the smaller is the habitat reaction. The plants in the experiment had the highest growth rate at pH 3.5 - 5.5. Plotting on the same scale the line for correlation of plant length and habitat reaction and the line for decreasing plant length with the increasing pH of water in experiment 1 (Fig. 3 a, b - diagrams) we can see that they are similar.
Humic acids, in which carbon is 90% of carbon in organic matter in the water of habitats examined, have a strong influence on plant growth. The remaining part of carbon is from the relatively not very active in plant life bounded and slow-reducing sugar, fatty acids, esters, alcohols, aminoacids, etc. The effect of this part of organic matter on the growth of A. vesiculosa can be explained as a supply of some food components and first of all the indispensable growth substances (B a d a r a 1965). Whereas the positive effect of humic acids may take the form of: (1) neutralization of the surphus of calcium ions (N i k 1 e w s k i 1968, S k i n d e r 1981) unfavourable for the growth of Aldrovanda, (2) facilitating the penetration of some ions into plant tissues by means of absorption of rations by humic acids as a colloidal system (L h o t s k y 1960), (3) forming complexes with metals, which may facilitate the penetration of some multivalent ions (G u m i ń s k i et al. 1956, Czerwiński 1967, K y ć 1968), (4) changing the permeability of cytoplasmatic membranes (G u m i ń s k i 1950, Rypaček 1962).
The optimum humic acid content for the growth of Aldrovanda is 5000-7000 microg C . l-1 of water (experiment 1) as confirmed by positive correlations between plant growth and the humic acid content in the interval 1870-6330 microg C . l-1. Concentrations of humic acids exceeding 10000 microg C . l-1 of water have an unfavourable effect on the growth of plants examined.
Because of the semi-heterotrophic way of life of these plants it is difficult to determine the effect of contents of particular macroelements on their growth. This is especially so for nitrogen and phosphorus (S c h m u c k e r and L i n n e m a n 1959). The experiment has been unsuccessful. But the correlations obtained show a positive effect of magnesium, sodium and iron ions and to a smaller extent of nitrogen ions on the individual and group characters and a negative effect of potassium, calcium and sulphur. With consideration to the contents of macroelements in plants examined only potassium, magnesium and sodium contents are positively correlated with the characters examined. Phosphorus, nitrogen, sulphur and calcium contents in plants are negatively correlated first of all with plant length and to a smaller extent with the biotic potential. It is interesting that the plant length is negatively correlated with nitrogen and phosphorus contents in plants. Thus it can be assumed that these elements in the habitat also affect negatively the plant growth, because usually their content in plants is higher the more of them is in the habitat (G a u d e t 1974). This paradox can be explained. As it has been said A. vesiculosa became adapted to life in habitats with slightly acid water abundant in humic acids and poor in inorganic food components. In habitats with more nitrogen and phosphorus in water, i.e., in more eutrophic ones, there are more microorganisms. Passive catching of zooplankton means its greater availability for plants. This may cause an increase of these elements in plants. Simultaneously humic acids contents decrease in these habitats and the water reaction increases. Changes of these factors, which have the strongest effect, are unfavourable for plants and decrease their growth rate. In such cases the correlation is negative.
Frequently the nutrient contents in plants depend on their contents in the habitat. They influence greatly the individual and group character. In case of A. vesiculosa such a positive relation to iron was observed. However, one should be careful, as the correlation may be due to accumulation of oxidized iron compounds (oxides and hydroxides) in plants. Especially as the correlation has not been confirmed in the following year. Worth mentioning are positive correlations between potassium, calcium and sulphur contents in plants and in water, although at a significance level α > 0.05. Similar relations have been recorded for other plants, amongst others, by Prędota-Twarda (1979) and Stebnicka (1979).
Calcium in a plant is an antagonist of potassium. In habitats examined potassium content increases with the decreasing calcium content and the simultaneous correlations do not exclude themselves. Their probability is greater as they were repeated in two consecutive years.
Thus, decreasing contents of elements in water of habitats examined, resulted in their smaller contents in plants in 1981, consistently with B o y d (1970) and G a u d e t (1974). It is more difficult to explain why there was some 25% of nitrogen and 18% of phosphorus less in plants as the content of these elements in carnivorous plants usually is not correlated with their content in the habitat. These elements occur more abundantly in young plants (Dykyjova 1973) or in the youngest plant organs (G u m i ń s k i 1977, C z e r w i ń s k i 1980). Thus it can be assumed that in 1981 the plants examined were older than in 1980. This is indicated by data from the Institute of Meteorology and Water Economy and by observations of naturalists and farmers, who observed a 2-4-week delay of vegetation season in 1980 as compared with 1981. Also the greater iron content in plants in 1981 confirms this thesis (possibility of accumulating more iron compounds in older plants). The generally high phosphorus content in relation to other elements and plants can be explained only by its accumulation in A. vesiculosa.
5. CONCLUSIONS
(1) Aldrovanda vesiculosa is a species having a quite broad phytosociological amplitude (it occurs in different plant associations and thus has various relations with the abundant group of macrohydrophytes), whereas as regards the chemistry of the habitat it is rather a stenotopic species.
(2) Best conditions for its growth are in water bodies having coenotic relations and water chemistry characteristic of eutrophic-dystrophic habitats with a proper ratio of inorganic substances and primarily of calcium to organic substances.
(3) Chemical factors having the greatest influence on the growth of A. vesiculosa are habitat reaction, contents in water of humic acids, calcium, magnesium and sodium.
(4) Habitat differentiation from the point of water chemistry and bottom sediments corresponding largely to A. vesiculosa population differentiation in individual and group characters shows not only the significant effect of water chemistry on growth and ecological organization of the population, but that changes in physico-chemical and biotic conditions of the habitat are the main cause of the disappearance of this plant.
(5) Active protection of A. vesiculosa should be first of all against the degradation of its habitats and biotopes and if not possible the species should be transferred to suitable natural habitats or to cultures in botany gardens.
(6) Substitute habitats of A. vesiculosa should have physical and biotic properties as in habitats 6 and 7, whereas 1 litre of water should contain: 0.3 - 0.6 mg of nitrates, 1.0-1.5 mg ammonia, over 0.06 mg phosphates, 2.4-4.0 mg potassium, less than 40 mg calcium, 6.0-15.0 mg magnesium, 8.0-13.0 mg sodium, 0.5 -1.0 mg iron, less than 25 mg sulphates, 5.0-12.0 mg chlorides, 5000-8000 microg C organic matter including about 4000-7000 microg C humic acids at pH about 5.
6. SUMMARY
On 10 localities of A. vesiculosa all over Poland (Fig. 1) its ecology was investigated, i.e., physico-chemical conditions and coenotic relations, population differentiation from the point of individual and group characters, finding whether these differences are caused by edaphic factors of the habitat, and ecological amplitude of the species.
Coenotic relations were determined on the basis of analysis of phytosociological records of plant patches where Aldrovanda occurs (Table 1). The species has a rather broad phytosociological amplitude having a mosaic character of occurrence within one habitat. Under conditions examined it is a component of plant associations belonging to two phytosociological classes (Potamogetonetea, Phragmitetea) occurring at turning into peat shores of water bodies. It seems that some plants most frequently accompanying A. vesiculosa have a great influence on its growth and development.
The trophic state of habitats was determined by finding the contents of organic matter, calcium and silicates in surface layers of bottom sediments, water reaction and hardness, its contents of macroelements, organic matter and humic acids. The habitats were differentiated in respect of characters examined (Tables 2, 3), i.e., from eutrophic ones (habitats 1, 7, 8) through various intermediate stages including alloitrophic to highly dystrophic (habitats 3, 8). According to criteria of P a t a 1 a s (1960b) the waters of these habitats are not very abundant in nitrates, averagely abundant in potassium, averagely-highly abundant in phosphates, ammonia and iron, and differentiated in contents of calcium, organic matter and humic acids. The contents of other macroelements are low. Although the chemistry of waters in which Aldrovanda occurs varies and is significantly differentiated, this variability is not great and the ecological scale is narrow compared with other macrohydrophytes.
'The best for growth and development of Aldrovanda are water bodies having vegetation composition and water chemistry characteristic of eutrophic-dystrophic habitats with a proper ratio of mineral substances (mainly calcium) to organic ones. A greater disturbance of this equilibrium may result in the disappearance of A. vesiculosa. Its disappearance from the majority of habitats of the Łęczna-Włodawa Lake District seems to prove this.
Chemical differentiation of habitats (also in time) corresponds with differentiation of individual and group characters of plants. The correlations (Tables 6, 7) and results of experiments (Table 8, Fig. 3) show that water reaction, its contents of humic acids and organic matter have the greatest influence on characters examined, whereas the influence of macroelements is smaller. Most distinct is the negative effect of calcium and the positive one of magnesium, sodium and probably iron. It is difficult to determine the effect of nitrogen and phosphorus because of the semi-heterotrophic way of feeding of A. vesiculosa.
Characters which show best the influence of chemical factors of the habitat and the ecological constitution of A. vesiculosa are plant length, number of shoots, leaves length, plant biomass and index of biotic potential of plants. And they should be used mainly in estimations and bioindication of deformations of its habitats.
Also positive correlations were observed between the contents of particular macroelements in water and in plants. This concerned potassium, sulphur and calcium. In the case of iron this correlation should be considered very carefully. The contents of particular elements in A. vesiculosa (Table 5) did not exceed the minimum and maximum (with the exception of phosphorus) given by different authors for other macrohydrophytes and approximated those in Utricularia sp. having a similar biology and occupying a similar ecological niche.
7. POLISH SUMMARY
Badania, przeprowadzone w 10 stanowiskach Aldrovanda vesiculosa położonych na terenie całej Polski (rys. 1), miały na celu poznanie jej ekologii, tj. warunków fizyczno-chemicznych i stosunków oenotycznych, w jakich ona występuje, zróżnicowania populacji pod względem cech osobniczych i grupowych, określenie, na ile różnice te powodowane są czynnikami edaficznymi siedliska oraz amplitudy ekologicznej gatunku.
Stosunki cenotyczne określono na podstawie analizy zdjęć fitosocjologicznych wykonanych w placach roślin, wśród których występuje Aldrovanda (tab. 1). Stwierdzono, że jest ona gatunkiem o dość szerokiej amplitudzie fitosocjologicznej, a w obrębie jednego siedliska z reguły występuje w układzie mozaikowym. W konkretnych warunkach jest komponentem zbiorowisk roślinnych należących do dwóch klas fitosocjologicznych (Potamogetonetea, Phragmitetea) występujących przy podtorfionych brzegach zbiorników wodnych. Wydaje się, że niektóre rośliny najczęściej towarzyszące A. vesiculosa mają silny wpływ na jej wzrost i rozwój.
Trofizm siedlisk określono oznaczając zawartość materii organicznej, wapnia i krzemianów w powierzchniowych warstwach osadów dennych, odczyn i twardość wody, zawartość w niej makroelementów, substancji organicznej i kwasów humusowych. Wyniki wskazują na zróżnicowanie siedlisk pod względem badanych cech (tab. 2, 3), co pozwala wyróżnić siedliska o różnym stopniu trofizmu, tj. od eutroficznych (siedliska 1, 7, 8), poprzez różne stadia pośrednie z alloitroficznymi włącznie, aż do silnie dystroficznych (siedliska 3, 8).
Według kryteriów P a t a 1 a s a (1960b) wody z badanych siedlisk są niezbyt zasobne w ażotany, średnio zasobne w potas, średnio-wysoko zasobne w fosforany, amoniak i żelazo, a zróżnicowane pod względem zawartości wapnia, substancji organicznej i kwasów humusowych. Zawartość pozostałych makroelementów jest niska. Pomimo że chemizm wód, w których występuje Aldrovanda, jest zmienny i istotnie zróżnicowany, to zmienność ta nie jest duża, a skala ekologiczna A. vesiculosa, w porównaniu do inńych makrohydrofitów, jest wąska.
Optymalnymi dla jej wzrostu i rozwoju są zbiorniki wodne charakteryzując się stosunkami cenotycznymi oraz chemizmem wody właściwym dla siedlisk eutroficzno-dystroficznych, w których występuje odpowiedni stosunek substancji mineralnych, a przede wszystkim wapnia, do substancji organicznych. Większa zmiana takiego układu równowagi może doprowadzić do wyginięcia A. vesiculosa. Za taką interpretacją przemawia fakt jej ustąpienia z większości siedlisk na Pojezierzu Łęczyńsko-Włodawskim.
Zróżnicowaniu chemicznemu siedlisk (także w czasie) odpowiada zróżnicowanie cech osobniczych i grupowych roślin. Stwierdzone korelacje (tab. 6, 7) i wyniki doświadczeń (tab. 8, rys. 3) wykazują, że największy wpływ na wielkość badanych cech ma odczyn wody, zawartość w niej kwasów humusowych i mateńi organicznej, mniejszy zaś makroelementów. Wśród nich najbardziej widoczny jest negatywny wpływ wapnia, a pozytywny magnezu, sodu i prawdopodobnie żelaza. Wpływ azotu i fosforu jest trudny do ustalenia z powodu na wpół heterotroficznego sposobu odżywiania się A. vesiculosa.
Do cech najlepiej wyrażających wpływ chemicznych czynników siedliska i konstytucję ekologiczną A. vesiculosa należy długość roślin, ich rozkrzewienie wyrażone liczbą pędów bocznych, długość liści, biomasa roślin oraz wskaźnik potencjału biotycznego roślin. One głównie powinny służyć do oceny i bioindykacji odkształceń jej siedlisk.
Stwierdzono także dodatnie korelacje pomiędzy zawartością poszczególnych makroelementów w wodzie i w roślinach. Dotyczy to potasu, siarki i wapnia. W przypadku żelaza uzyskaną korelację należy traktować z dużą ostrożnością. Zawartość poszczególnych pierwiastków w roślinach A. vesiculosa (tab. 5) nie przekracza zawartości minimalnych i maksymalnych (z wyjątkiem fosforu), podanych przez różnych autorów dla innych makrohydrofitów, a jest bardzo zbliżona do ich zawartości w Utricularia spp. charakteryzujących się podobną biologią i zajmujących podobną niszę ekológiczną.
8. REFERENCES
1. A s h i d a J. 1934 - Studies on the leaf movement of Aldrovanda vesiculosa L. I. Process and mechanism of the movement - Mem. Coll. Sci. Kyoto Univ., ser. B, 9: 141-244.
2. A s h i d a J. 1935 - Studies on the leaf movement of Aldrovanda vesiculosa L. II. Effects of mechanical, electrical, thermal, osmotic and chemical influences - Mem. Coll. Sci. Kyoto Univ., ser. B, 11: 55-113.
3. B a b k o A. K., P i 1 i p i e n k o A. 1955 - Analiza kolorymetryczna [Colorimetric analysis] - Państwowe Wydawnictwo Techniczne, Warszawa, 340 pp.
4. B a d u r a L. 1965 - O mechanizmie "stymulującegó” wpływu humianu sodu na proces fermentacji alkoholowej i rozmnażania drożdży [The "stimulating" effect of humic natrium on alcoholic fermentation and yeast growth] - Acta Soc. Bot. Pol. 34: 287-328.
5. B a r r o u s H. L., S i m p s o n E. C. 1962 - An EDTA method for the direct routine determination of calcium and magnesium in soil and plant tissue - Soil. Sci. Am. Proc. 26: 443-445.
6. B e r n a t o w i c z S., W o 1 n y P. 1974 - Botanika dla limnologów i rybaków [Botany for limnologists and fishermen] - Państwowe Wydawnictwo Rolnicze i Leśne, Warszawa, 518 pp.
7. B o y d C. E. 1968 - Some aspects of aquatic plant ecology - Reservoir Fishery Resources Symposium, Athens, April 5-7, Athens, Georgia, Washington, D. C., Am. Fish. Soc. 114- 129.
8. B o y d C. E. 1970 - Chemical analyses of some aquatic plants - Arch. Hydrobiol. 67: 78-85.
9. B u c k m a n H. C., B r a d y N. C. 1971 - Gleba i jej właściwości [Soil and its properties] - Państwowe Wydawnictwo Rolnicze i Leśne, Warszawa, 529 pp.
10. Buczek J., Burzyński M., Jurajda K., Kubik-Dobosz G., Lisiak M.J., S t a b r o w s k a J., S l e s a k E., T a t k o w s k a E. 1976 - Ćwiczenia z fizjologii roślin [Exercises in plant physiology] - Wyd. Uniwersytetu Wrocławskiego, Wroclaw, 187 pp.
11. C z e r w i ń s k i W. 1967 - Znaczenie humianu w kulturach wodnych pod kątem widzenia czynnika minimum [Significance of humic acid in aquatic cultures from the point of minimum factor] - Acta Soc. Bot. Pol. 36: 185-206.
12. C z e r w i ń s k i W. 1980 - Fizjologia roślin [Plant physiology] - Państwowe Wydawnictwo Naukowe, Warszawa,. 604 pp.
13. D y k y j o v a D. 1973 - Accumulation of mineral nutrients in the biomass of reedswamp species (In: Ecosystem study on wetland biome in Czechoslovakia, M. S. Hejny) - Czechoslovak IBP PT-PP, Report No. 3, Czechoslovak Academy of Sciences, l51 – l6l.
14. D y k y j o v a D. 1979 - Selective uptake of mineral ions and their concentration factors in aquatic higher plants - Folia Geobot. Phytotax., Praha, 14: 267 - 324.
15. E l a n d t R. 1964 - Statystyka matematyczna w zastosowaniu do doświadczalnictwa rolniczego [Mathematical statistics in agricultural experiments] - Państwowe Wydawnictwo Naukowe, Warszawa, 595 pp.
16. F i j a ł k o w s k i D. 1958a - Obserwacje nad ekologią i rozmieszczeniem wierzby borówkolistnej (Salix myrtilloides L.) na Pojezierzu Łęczyńsko-Włodawskim [Observations on the ecology and distribution of Salix myrtilloides L. on the Łęczna-Włodawa Lake District] - Acta Soc. Bot. Pol. 27: 605 - 612.
17. F i j a ł k o w s k i D. 1958b - Badania nad rozmieszczeniem i ekologią aldrowandy pęcherzykowatej (Aldrovanda vesiculosa L.) na Pojezierzu Łęczyńsko-Włodawskim [Studies on the distribution and ecology of Aldrovanda vesiculosa L. on the Łęczna-Włodawa Lake District] - Acta Soc. Bot. Pol. 27: 597- 604.
18. F i j a ł k o w s k i D. 1959 - Szata roślinna jezior Łęczyńsko-Włodawskich i przylegających do nich torfowisk [Vegetational cover of Łęczna-Włodawa lakes and the neighbouring peat bogs] - Ann. UMC-S, sectio B, 14: 131-206.
19. G a u d e t J. J. 1974 - The normal role of vegetation in water (In: Aquatic vegetation and its use and control, Ed. D. S. Mitchell) - UNESCO, Paris, 24-37.
20. G o m ó ł k a E., S z y p o w s k i W. 1975 – Cwiczenia laboratoryjne i rachunkowe w chemii wody [Laboratory and counting excercises in water chemistry] - Wydawnictwo Politechniki Wrocławskiej, lnstytut Inżynierii Ochrony Środowiska, 231 pp.
21. G u m i ń s k i S. 1950 – Badania nad warunkami i mechanizmem działania związków próchnicznych na organizm roślinny [Studies on the conditions and mechanism of the effect of humus compounds on plant organism] - Acta Soc. Bot. Pol. 20: 589-620.
22. G u m i ń s k i S. 1977 - Ogólna fizjologia roślin [General plant physiology] – Państwowe Wydawnictwo Naukowe, Warszawa - Wrocław, 449 pp.
23. G u m i ń s k i S., G um i ń s k a Z., Sulej J. 1965-Effects of humate,agar-agar and EDTA on the development of tomato seedlings in aerated and non aerated water cultures - J. exp. Bot. 16: l5l - 162.
24. H e g i G. 1961 - Illustrierte Flora von Mittel Evropa - Band IV/2 Teil, Teilband A., Carl Hanser Verlag, München, 497-1112.
25. H e l l e r R. 1953 - Recherches sur le nutrition minerale des tissues vegetaux cultives "in vitro" - Ann. Sci. Nat. Bot. 14: 1-223.
26. Hermanowicz W., Dożańska W., Sikorowska C., Kelus J. 1967 - Fizyczno-chemiczne badania ścieków miejskich i osadów ściekowych [Physico-chemical investigations of municipal sewage and sludge] - Arkady, Warszawa, 846 pp.
27. H u t c h i n s o n G. E. 1975 - A treatise on limnology. Vol. III. Limnological botany - A. Wiley-Intersc. Public., New York- London-Sydney-Toronto, 660 pp.
28. K a m i ń s k i R. 1983 - Aldrowanda pęcherzykowata (Aldrovanda vesiculosa L.) ginącą rodliną w Polsce [Aldrovanda vesiculosa L. a vanishing plant in Poland] - Chrońmy Przyr. ojcz. 8: 20-24.
29. K a m i ń s k i R. 1987 - Studies on the ecology of Aldrovanda vesiculosa L. II. Organic substances, physical and biotic factors and the growth and development of A. vesiculosa - Ekol. pol. 35: 591-609.
30. K a n o n o w a M. 1968 - Substancje organiczne gleby. Ich budowa, właściwości i metody badań [Organic substances of soil - structure, properties and methods of investigations] - Państwowe Wydawnictwo Rolnicze i Leśne, Warszawa, 392 pp.
31. K r a w i e c o w a A., K u c z y ń s k a I. 1964 - Roślinność rezerwatu Łężczak pod Raciborzem [Vegetation of the Łężczak reserve near Raciborz] - Acta Univ. Wratislav., Prace Bot. 24: 5-31.
32. K y ć S. 1968 - Próba wyjaśnienia mechanizmu działania humianu sodowego na przyrost ilości komórek i suchej masy Scenedesmus quadricauda (Turp.) Breb. [An attempt to explain the effect of humic natrium on the number of cells and dry weight of Scenedesmus quadricauda (Turp.) Breb.) - Ph.D. Thesis, Chair of Plant Physiology, University of Wrocław.
33. L h o t s k y S. 1960 - O wpływie próchnic na glony [The effect of humus on algae] - Acta Agrobot. 9: 113-116.
34. L i t y ń s k i T., J u r k o w s k a H., G o r I a c h E. 1972 - Analiza chemiczno-rolnicza. Gleby i nawozy [Chemical and agricultural analysis. Soils and fertilizers] - Państwowe Wydawnictwo Naukowe, Warszawa-Kraków, 178 pp.
35. L u r i e Ju. Ju. 1973 - Unificirovannye metody analiza vod - Izd. Nauka, Moskva, 377 pp.
36. M a t u s z k i e w i c z W. 1967 - Przegląd systematyczny zbiorowisk roślinnych Polski [Systematical review of plant communities in Poland] (In: Wstęp do fitosocjologii praktycznej, Ed. A. Scamoni) - Państwowe Wydawnictwo Rolnicze i Leśne, Warszawa, 175-229.
37. M a t u s z k i e w i c z W. 1982 - Przewodnik do oznaczania zbiorowisk roślinnych Polski [Key to plant communities in Poland] - Państwowe Wydawnictwo Naukowe, Warszawa, 298 pp.
38. M a z r i m a s J. 1974 - Further hints on growing Aldrovanda - Carnivor. Pl. Newslet. 3: 27-28.
39. M i c h a l a k S. 1963 - Staw Nowokuźnicki - rezerwat na Śląsku Opolskim [Nowokuźnicki pond - a reserve in Silesian Opole] - Chrońmy Przyr. ojcz. 19: 24-27.
40. N i k l e w s k i B. 1968 - Torf a roślina [Peat and plant] - Wiad. bot. 12: 211-228.
41. N o w o s i e 1 s k i O. 1968 - Metody oznaczania potrzeb nawożenia [Methods for determining the fertilization demands] - Państwowe Wydawnictwo Rolnicze i Leśne, Warszawa, 667 pp.
42. P a n f i l J. 1978 - Pojezierze Mazurskie. Z serii Przyroda Polska [Masurian Lakeland. From the series Polish Nature] - Wiedza Powszechna, Warszawa, 186 pp.
43. P a t a l a s K. 1960a - Stosunki termiczne i tlenowe oraz przeźroczystość wody w 44 jeziorach okolic Węgorzewa [Thermal and oxygen conditions and water transparency in 44 lakes of the Węgorzewo surroundings] - Roczn. Nauk roln. B, 77: 105-209.
44. P a t a l a s K. 1960b - Charakterystyka składu chemicznego wody z 44 jezior okolic Węgorzewa [Characteristics of chemical composition of water from 44 lakes of the Węgorzewo surroundings] Roczn. Nauk roln. B. 77: 243-297.
45. P i p e r C. S. 1957 - Analizy gleb i roślin [Analyses of soil and plants] - Państwowe Wydawnictwo Naukowe, Warszawa, 420 pp.
46. P r ę d o t a - T w a r d a B. 1979 - Ekologia Salvinia natans (L.) All. na Dolnym Śląsku [Ecology of Salvinia natans (L.) All. in Lower Silenia] - Ph.D. Thesis, Institute of Botany, University of Wrocław.
47. R a b o t n o v T. 1950 - Źiznennyj cikl mnogoletnich travjanistych rastenij w lugovych cenozach - Geobotanika, 3: 7-204.
48. R a d w a n S., P o d g ó r s k i W., K o w a 1 c z y k C. 1974 - Charakterystyka jezior Pojezierza Łęczyńsko-Włodawskiego na podstawie abiotycznych czynników środowiskowych [Characteristics of lakes of the Łęczna-Włodawa Lake District on the basis of abiotic environmental factors] - Ann. UMC-S, sectio C, 29: 231-246.
49. R a o C. R. 1965 - Linear statistical inference and its applications - A. Wiley-Intersc. Public., New York - London - Sydney - Toronto, 522 pp.
50. R y p a č e k V. 1962 - Der Einfluss isolierter Humusstoffe auf einige physiologische Ausserungen der Pflanzenzelle (In: Studies about humus) - Published by Research Institute of Crop Production, Prague, 235-243.
51. S c h m u c k e r T., L i n n e m a n G. 1959 - Carnivorie (ln: Handbuch der Pflanzenphysiologie: Ed. W. Ruhland, Bd. Xl. Heterotrophie) - Springer-Verlag, Berlin-Gőttingen-Heidelberg, 198-283.
52. S c u 1 t h o r p e C. D. 1971 - The biology of aquatic vascular plants - Ed. Edward Arnold, London, 630 pp.
53. S k i n d e r N. W. 1981 - Hamujące działanie humianu sodowego na liczebność populacji Oscillatoria sancta (Kutzing) Gomont [Inhibiting effect of humic natrium on the abundance of Oscillatoria sancta (Kutzing) Gomont population] - Acta Univ. Wratislav., Prace Bot. 26: 3-58.
54. S ł o d c z y k K. 1984a - Zdolność produkcyjna siedlisk Iris pseudoacorus L. [Productivity of habitats of Iris pseudoacorus L.] - Zesz. nauk. WSI w Opolu, Seria: Problematyka różna, 7: 103-120.
55. S ł o d c z y k K. 1984b - Wplyw trofizmu siedlisk na rozwój roślin Glyceria aquatica (L.) Wahlnb. w populacjach naturalnych [The influence of trophic state of habitats on the growth of Glyceria aquatica (L.) Wahlnb. in natural populations] - Zesz. nauk. WSI w Opolu, Seria: Problematyka różna, 7: 121-137.
56. S o k o ł o w s k i A. 1972 - Roślinność rezerwatu "Perkuć” w Puszczy Augustowskiej [Vegetation of the "Perkuć" reserve in the Augustów Forest] - Chrońmy Przyr. ojcz. 28: 68-73.
57. S t a r m a c h K., W r ó b e l S., P a s t e r n a k K. 1978 - Hydrobiologia, limnologia [Hydrobiology, limnology] - Państwowe Wydawnictwo Naukowe, Warszawa, 620 pp.
58. S t e b n i c k a E. 1979 - Studia nad ekologią trzech gatunków rdestnic Potamogeton natans L., Potamogeton lucens L., Potamogeton crispus L. [The ecology of three species of pond-weeds Potamogeton natans L., Potamogeton lucens L. and Potamogeton crispus L.] - Ph.D. Thesis, Institute of Botany, University of Wrocław.
59. S z a f e r W. 1972 - Szata roślinna Polski [Vegetation cover of Poland] - Państwowe Wydawnictwo, Naukowe, Warszawa, Vol. 1, 2, 960 pp.
60. U r a n o v A. A. 1960 - Žiznennoe sostojanie vida w rastitelnom soobščestvie - Bjul. mosk. Obšč. Ispyt. Prir., Biol. 65: 1-190.
61. W e n d t A. 1952-1958 - Die Aquarienpflanzen in Wort und Bild - Alfred Kernen Verlag, Stuttgart, 338 pp.
62. W o ż a k o w s k a - N a t k a n i e c H. 1976-1980 - Dynamika populacji roślin wodnych i błotnych Dolnego Śląska w warunkach naturalnych i antropopresji [Dynamics of aquatic and uliginose plant populations under natural conditions and anthropopressure] - Umowa Nr. 484/1/OB/1976-80 (manuscript).
63. Z i e 1 i ń s k i R. 1972 - Tablice statystyczne [Statistical tables] - Państwowe Wydawnictwo Naukowe, Warszawa, 392 pp.
64. Ż u k o w s k i W. 1963 - Notatki florystyczne z Wielkopolski [Floristic notes from Great Poland] - Fragm. Flor. Geobot. 9: 463-467.
(Received 29 October 1986)
Table 1. Plant aggregates with Aldrovanda vesiculosa (see Table 1 on separate page)
Copyright (c) 1995-2002 www.BestCarnivorousPlants.com